This year, the Gates Foundation will begin an observational study that could identify correlates of protection (COP) for multiple pathogens. The START Center was approached by the Gates Foundation Vaccine Development team to develop recommendations for this study, and, in particular, to help with the sample size required for this study.
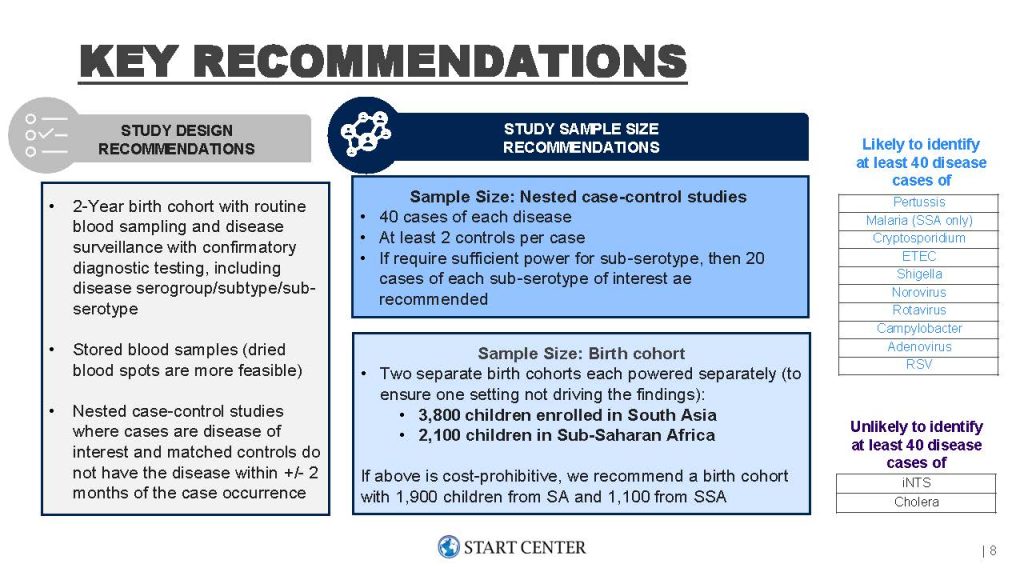
The START team provided recommendations for the study design based off a rapid literature review of other observational COP studies and consultation from experts from the Fred Hutch and the Gates Foundation client team. The START team recommended a two-year birth cohort with nested case-control studies per pathogen, each with a sample size of 40 cases and a 1:2 case:control ratio. To achieve these sample sizes for the majority of pathogens that the Gates team was interested in, the START team recommended a birth cohort size of 2,100 in Sub-Saharan Africa and 3,800 in South Asia. The case-control study sample size was powered using the CoRpower calculator developed by content experts from the Fred Hutch. The birth cohort sizes were primarily determined by pulling publicly available IHME incidence estimates. The START team also collated key considerations for the study design that would need to be addressed, including controlling influential confounders, definitions for controls, blood sampling techniques, and cohort sizing using non-IHME incidence estimates.