Although dengue is the fastest growing vector-borne disease globally, there are limited effective prevention and control strategies. There are nearly 100 million annual cases, and more than 6 billion people will be at risk by 2080. Similarly, despite promising interventions, we are far from achieving global malaria elimination. There are nearly 230 million annual malaria cases, and 400,000 deaths, 67% of which occur in children under 5. Given the substantial current and future threat of vector-borne diseases, novel biotechnological innovations have been developed in which mosquitoes, biologically modified to alter disease transmission, are released to suppress or replace existing mosquito species. Examples of such interventions include Wolbachia, a naturally occurring endosymbiotic bacteria, which has been shown to compete with viruses such as Dengue, Zika, Chikungunya and yellow fever, which has been shown to be effective, cost-effective, and acceptable by communities across study settings. Similar technologies are currently being developed for malaria, such as gene drive. The gene drive approach aims to produce genetically modified Anopheles mosquitoes that pass genetic modifications to their offspring, reducing the number of female mosquitoes which can cause malaria.
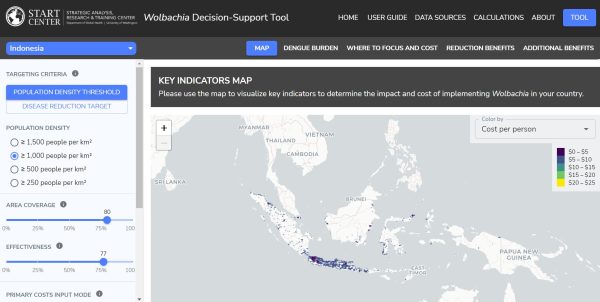
What is not known to date is how to best implement a mosquito-targeted approach at scale across technologies, diseases, and geographies. There is a need to better understand costs and benefits. Without evidence on the estimated costs and benefits, there is a risk that mosquito release programs will not be implemented in areas where there is the greatest need and potentially the greatest payoff for the investment. This knowledge gap was addressed in the development of a cost-benefit tool. This tool is used for forecasting costs and benefits of existing methods in new geographies, and of novel gene drive interventions that will be field-tested in the future.
There were three major aspects of the project strategy. Firstly, the team met with experts and conducted desk review to identify key cost drivers and discuss important considerations for the cost-benefit tool and developed a white paper based on this research. Secondly, the team focused their efforts on extracting data from modeled dengue/malaria burden and population rasters using administrative boundary shapefiles. They collected data on secondary inputs (e.g., cost of illness, healthcare utilization), developed datasets, and verified assumptions and decisions with experts and review of academic literature. Lastly, the team put all the assumptions, data, and calculations together into an interactive R Shiny application for dengue in Brazil, Colombia, Indonesia, Mexico, Sri Lanka, and Vietnam, and for malaria in Burkina Faso.
The team developed the tool in a web-based R shiny application to ensure that it could be accessible and understandable to mosquito release experts (i.e., mosquito rearing organizations) and non-experts (i.e., policymakers in country). The team worked with a consultant for the Gates Foundation to make sure all of the code would be posted on Github, where other users could access and update it in the future.
Access the updated tool here.





